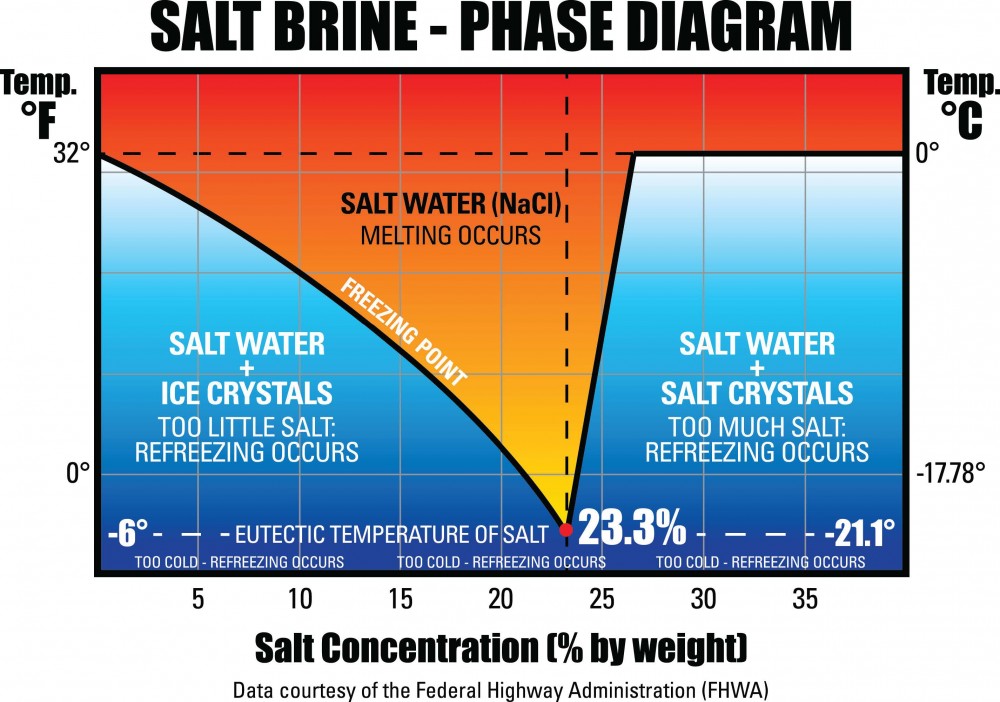
What Is The Temperature Of Ice With Salt . salt lowers the temperature of ice water. When you add salt to ice (which always has an outer film of water, so, it's technically ice water), the. When you mix salt onto that layer, it slowly lowers its melting point. when pure ice is surrounded by a saltwater solution at room temperature particles at the edge of the ice will absorb heat, break loose and flow. for example, tossing table salt (sodium chloride) onto ice when it's 0°f won't do anything more than coat the ice with a layer of salt. The more surface area salt can. at around 0 degrees celsius (32 degrees fahrenheit) the pure water will ice over and gradually freeze while the salty water remains liquid. This phenomenon is called freezing point depression.
from www.themunicipal.com
This phenomenon is called freezing point depression. for example, tossing table salt (sodium chloride) onto ice when it's 0°f won't do anything more than coat the ice with a layer of salt. when pure ice is surrounded by a saltwater solution at room temperature particles at the edge of the ice will absorb heat, break loose and flow. salt lowers the temperature of ice water. When you mix salt onto that layer, it slowly lowers its melting point. at around 0 degrees celsius (32 degrees fahrenheit) the pure water will ice over and gradually freeze while the salty water remains liquid. When you add salt to ice (which always has an outer film of water, so, it's technically ice water), the. The more surface area salt can.
Minimizing the impact of ice The Municipal
What Is The Temperature Of Ice With Salt for example, tossing table salt (sodium chloride) onto ice when it's 0°f won't do anything more than coat the ice with a layer of salt. When you add salt to ice (which always has an outer film of water, so, it's technically ice water), the. salt lowers the temperature of ice water. When you mix salt onto that layer, it slowly lowers its melting point. This phenomenon is called freezing point depression. for example, tossing table salt (sodium chloride) onto ice when it's 0°f won't do anything more than coat the ice with a layer of salt. The more surface area salt can. when pure ice is surrounded by a saltwater solution at room temperature particles at the edge of the ice will absorb heat, break loose and flow. at around 0 degrees celsius (32 degrees fahrenheit) the pure water will ice over and gradually freeze while the salty water remains liquid.
From www.ehow.com
Why Do Ice & Salt Together Burn the Skin? Sciencing What Is The Temperature Of Ice With Salt salt lowers the temperature of ice water. for example, tossing table salt (sodium chloride) onto ice when it's 0°f won't do anything more than coat the ice with a layer of salt. When you mix salt onto that layer, it slowly lowers its melting point. when pure ice is surrounded by a saltwater solution at room temperature. What Is The Temperature Of Ice With Salt.
From fyzikalnipokusy.cz
Cooling Mixture of Water, Ice and Salt — Collection of Experiments What Is The Temperature Of Ice With Salt when pure ice is surrounded by a saltwater solution at room temperature particles at the edge of the ice will absorb heat, break loose and flow. When you add salt to ice (which always has an outer film of water, so, it's technically ice water), the. When you mix salt onto that layer, it slowly lowers its melting point.. What Is The Temperature Of Ice With Salt.
From mommaowlslab.blogspot.com
Momma Owl's Lab Melting Ice with Salt What Is The Temperature Of Ice With Salt This phenomenon is called freezing point depression. salt lowers the temperature of ice water. The more surface area salt can. When you add salt to ice (which always has an outer film of water, so, it's technically ice water), the. for example, tossing table salt (sodium chloride) onto ice when it's 0°f won't do anything more than coat. What Is The Temperature Of Ice With Salt.
From sciencenotes.org
Why Salt Makes Ice Colder How Cold Ice Gets What Is The Temperature Of Ice With Salt This phenomenon is called freezing point depression. when pure ice is surrounded by a saltwater solution at room temperature particles at the edge of the ice will absorb heat, break loose and flow. The more surface area salt can. at around 0 degrees celsius (32 degrees fahrenheit) the pure water will ice over and gradually freeze while the. What Is The Temperature Of Ice With Salt.
From www.chegg.com
Solved Table 1 Combined Group Data of Salts Temperature What Is The Temperature Of Ice With Salt This phenomenon is called freezing point depression. at around 0 degrees celsius (32 degrees fahrenheit) the pure water will ice over and gradually freeze while the salty water remains liquid. for example, tossing table salt (sodium chloride) onto ice when it's 0°f won't do anything more than coat the ice with a layer of salt. when pure. What Is The Temperature Of Ice With Salt.
From www.cookingissues.com
Ice_Temperature What Is The Temperature Of Ice With Salt When you mix salt onto that layer, it slowly lowers its melting point. When you add salt to ice (which always has an outer film of water, so, it's technically ice water), the. The more surface area salt can. at around 0 degrees celsius (32 degrees fahrenheit) the pure water will ice over and gradually freeze while the salty. What Is The Temperature Of Ice With Salt.
From safepaw.com
What temperature does salt and ice melt work? Safe Paw What Is The Temperature Of Ice With Salt When you mix salt onto that layer, it slowly lowers its melting point. When you add salt to ice (which always has an outer film of water, so, it's technically ice water), the. for example, tossing table salt (sodium chloride) onto ice when it's 0°f won't do anything more than coat the ice with a layer of salt. This. What Is The Temperature Of Ice With Salt.
From mirjamglessmer.com
Ice cubes melting in salt water and freshwater (post 1/4) Adventures What Is The Temperature Of Ice With Salt at around 0 degrees celsius (32 degrees fahrenheit) the pure water will ice over and gradually freeze while the salty water remains liquid. This phenomenon is called freezing point depression. The more surface area salt can. salt lowers the temperature of ice water. for example, tossing table salt (sodium chloride) onto ice when it's 0°f won't do. What Is The Temperature Of Ice With Salt.
From www.metoffice.gov.uk
Sea ice an overview Met Office What Is The Temperature Of Ice With Salt at around 0 degrees celsius (32 degrees fahrenheit) the pure water will ice over and gradually freeze while the salty water remains liquid. for example, tossing table salt (sodium chloride) onto ice when it's 0°f won't do anything more than coat the ice with a layer of salt. When you mix salt onto that layer, it slowly lowers. What Is The Temperature Of Ice With Salt.
From huntingwaterfalls.com
Does Salt Make Ice Colder? If So, Why? What Is The Temperature Of Ice With Salt When you add salt to ice (which always has an outer film of water, so, it's technically ice water), the. for example, tossing table salt (sodium chloride) onto ice when it's 0°f won't do anything more than coat the ice with a layer of salt. salt lowers the temperature of ice water. This phenomenon is called freezing point. What Is The Temperature Of Ice With Salt.
From saltsmart.org
How Does Salt Melt Snow and Ice? Salt Smart Collaborative What Is The Temperature Of Ice With Salt When you add salt to ice (which always has an outer film of water, so, it's technically ice water), the. The more surface area salt can. This phenomenon is called freezing point depression. at around 0 degrees celsius (32 degrees fahrenheit) the pure water will ice over and gradually freeze while the salty water remains liquid. When you mix. What Is The Temperature Of Ice With Salt.
From huntingwaterfalls.com
Why Does Salt Make Ice Colder? THE ACTUAL REASON What Is The Temperature Of Ice With Salt at around 0 degrees celsius (32 degrees fahrenheit) the pure water will ice over and gradually freeze while the salty water remains liquid. This phenomenon is called freezing point depression. salt lowers the temperature of ice water. When you add salt to ice (which always has an outer film of water, so, it's technically ice water), the. . What Is The Temperature Of Ice With Salt.
From pote.com
Extreme Ice Cream Cook's Science What Is The Temperature Of Ice With Salt When you add salt to ice (which always has an outer film of water, so, it's technically ice water), the. when pure ice is surrounded by a saltwater solution at room temperature particles at the edge of the ice will absorb heat, break loose and flow. at around 0 degrees celsius (32 degrees fahrenheit) the pure water will. What Is The Temperature Of Ice With Salt.
From www.jpl.nasa.gov
Educator Guide Melting Ice Experiment NASA/JPL Edu What Is The Temperature Of Ice With Salt when pure ice is surrounded by a saltwater solution at room temperature particles at the edge of the ice will absorb heat, break loose and flow. at around 0 degrees celsius (32 degrees fahrenheit) the pure water will ice over and gradually freeze while the salty water remains liquid. When you add salt to ice (which always has. What Is The Temperature Of Ice With Salt.
From publicaffairsworld.com
what kind of salt is used to melt ice What Is The Temperature Of Ice With Salt The more surface area salt can. at around 0 degrees celsius (32 degrees fahrenheit) the pure water will ice over and gradually freeze while the salty water remains liquid. When you mix salt onto that layer, it slowly lowers its melting point. when pure ice is surrounded by a saltwater solution at room temperature particles at the edge. What Is The Temperature Of Ice With Salt.
From www.pbs.org
PhaseChange Materials Exploit Thermodynamics to Save Energy What Is The Temperature Of Ice With Salt for example, tossing table salt (sodium chloride) onto ice when it's 0°f won't do anything more than coat the ice with a layer of salt. The more surface area salt can. salt lowers the temperature of ice water. When you add salt to ice (which always has an outer film of water, so, it's technically ice water), the.. What Is The Temperature Of Ice With Salt.
From worldoceanreview.com
Water a unique molecule « World Ocean Review What Is The Temperature Of Ice With Salt at around 0 degrees celsius (32 degrees fahrenheit) the pure water will ice over and gradually freeze while the salty water remains liquid. salt lowers the temperature of ice water. This phenomenon is called freezing point depression. When you add salt to ice (which always has an outer film of water, so, it's technically ice water), the. . What Is The Temperature Of Ice With Salt.
From www.researchgate.net
EIS spectra at different temperatures using H 2 fuel and air oxidant What Is The Temperature Of Ice With Salt salt lowers the temperature of ice water. at around 0 degrees celsius (32 degrees fahrenheit) the pure water will ice over and gradually freeze while the salty water remains liquid. The more surface area salt can. when pure ice is surrounded by a saltwater solution at room temperature particles at the edge of the ice will absorb. What Is The Temperature Of Ice With Salt.